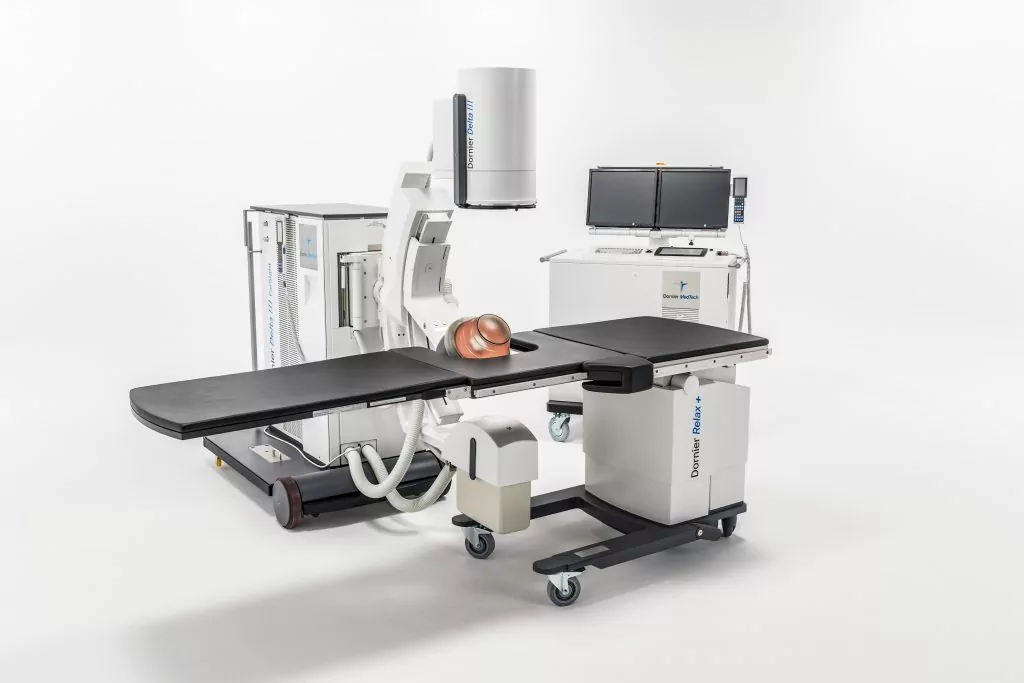
Advanced Kidney Stone Treatment Lithotripter Arrives In Japan
Dornier MedTech Brings the Most Advanced Kidney Stone Treatment Lithotripter—Dornier Delta® III—to Japan
Dornier MedTech, a global leader in innovative kidney stone management, has received PMDA (Pharmaceuticals and Medical Devices Agency) regulatory approval to market the Delta® III in Japan. This is the latest generation of the world’s best-selling and most clinically cited lithotripter, the Delta II.
The Delta III reflects Dornier’s continued commitment to develop the most cutting-edge solutions for kidney stone treatment. Compared to its predecessor, the Delta III provides improvements of:
- Powerful IMAGING (for improved stone visualization)
- Maximized ENERGY (to treat more stones in more patients)
- Enhanced EFFICIENCY (with time saving efficiencies to serve more patients)
Powerful IMAGING: the system provides improved imaging quality with 4 times1 more power and two imaging modalities (Ultrasound or X-ray) for better stone visibility.
Maximized ENERGY: Featuring Dornier’s exclusive Opticouple®, an optical camera system—visualized air bubbles are removed in the coupling gel at the interface of the therapy head and patient’s skin with a hand swipe. Clinical research2 shows a 43% increase in effectiveness with less shock wave energy needed per treatment.
Enhanced EFFICIENCY: Additionally, with OptiCouple, urologists can experience higher stone free rates3 and a 25% decrease in treatment time2, allowing doctors to see more patients per day to realize greater revenue.
Said Akira Kinoshita, GM, Dornier MedTech Japan, “About 1 in 10 people worldwide will have a kidney stone at some point in their lifetime, with more than 50% of patients experiencing one or more recurrences. The prevalence of stone diseases more than doubled in Japan during a 40 year period4, so our country’s launch of the popular Delta III lithotripter is a significant benefit not only for urologists but for kidney stone sufferers as well.”
Media Contact:
Kelly Westermann
+1 (404) 989-0498
Dornier MedTech is headquartered in Munich, Germany, and is a full subsidiary of Accuron MedTech. Dornier is a medical device company focused on providing leading technology and improving life by delivering scientifically superior products and solutions to physicians, healthcare providers and research groups involved in urological care. As pioneers of the lithotripsy and a variety of surgical lasers, Dornier’s 40 years of innovation and service has made it one of the most trusted MedTech companies in the industry.
1Compared to the Delta II.
2Tailly GG, Tailly-Cusse MM: “Optical Coupling Control: An Important Step Toward Better Lithotripsy”,
J Endourol (2014 28(11):1368-73).
3A new optical coupling control technique and application in SWL. Jian Lin Lv, Urolithiasis (2016) 44: 5349-544
4Romero V, Akpinar H, Assimos DG: “Kidney Stones: A Global Picture of Prevalence, Incidence, and Associated Risk Factors,” Rev Urol (2010 Spring-Summer; 12(2-3): e86–e96).
©2018 DORNIER MEDTECH. All rights reserved. Subject to change without notice. Delta and Opticouple are registered trademarks of Dornier MedTech. All subsequent uses of these brand names throughout this document are also protected.