NEWS
Dornier MedTech Expands Edge Medical® Partnership to Bring MP1000 Robotic Surgery System to Germany and Austria
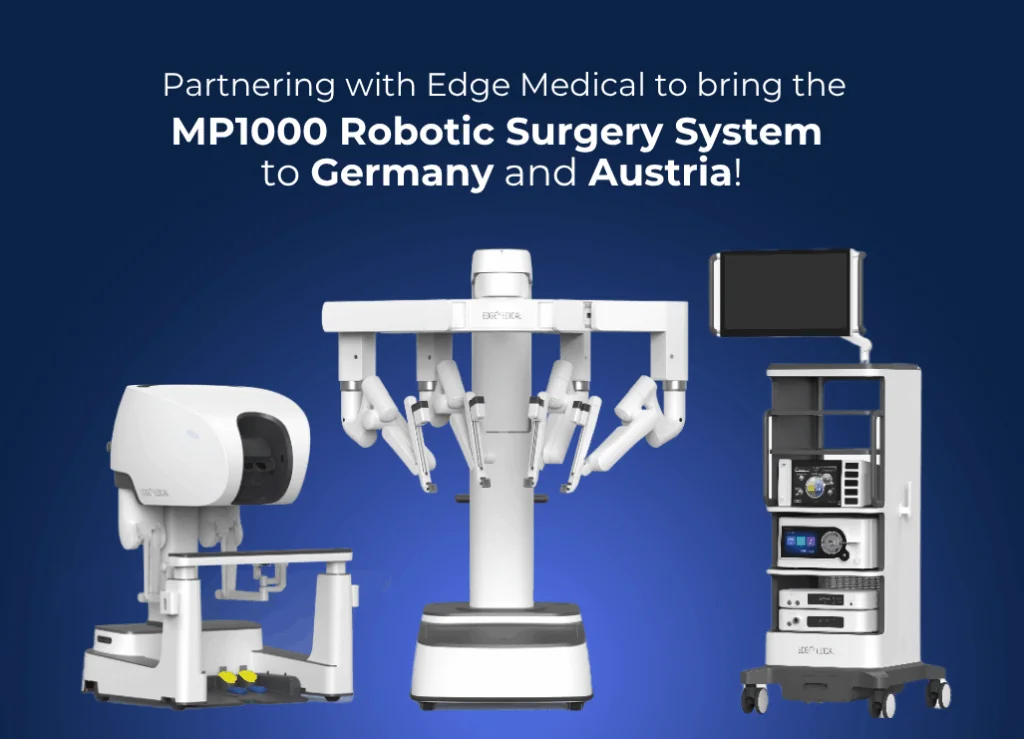
Munich, 5 November 2025 – Dornier MedTech (Dornier), a global leader in urological innovation, is extending its partnership with Edge Medical® to introduce the MP1000 Multi-Port Robotic Surgery System in Germany and Austria. This follows the successful rollout in Spain and Portugal earlier this year.
The expansion marks a major step in Dornier’s mission to accelerate robotic-assisted surgery adoption across Europe. It also aligns with Dornier’s strategy to become a comprehensive urological medical device provider globally, bringing more innovative robotic-assisted surgery solutions to surgeons and patients.
“Germany and Austria are among Europe’s most advanced healthcare markets. Launching MP1000 here is a milestone in our development strategy,” said Henrik Welbers, General Manager, Dornier MedTech Europe North. “We aim to equip hospitals and surgeons with cutting-edge robotic systems that elevate surgical precision and patient outcomes.”
Edge Medical’s CEO, Dr. Wang Jianchen, added: “Our collaboration with Dornier continues to gain momentum. With Dornier’s trusted network, we’re confident more providers will benefit from MP1000’s advanced technology and clinical support.”
About Edge Medical & the MP100
Headquartered in Shenzhen, China, Edge Medical is a global leader in surgical robotics innovation. Edge Medical is the world’s second company to master multi-port, single-port, and bronchoscopic robotic technologies. Edge Medical has an interdisciplinary R&D and manufacturing team of nearly 1,000 members, with employees and partners spanning over 60 countries.
The MP1000 combines innovative robotic algorithms with 3DHD visualization, delivering exceptional precision and flexibility for minimally invasive urological procedures such as radical prostatectomies. Its articulated arms mimic human dexterity, potentially enabling complex surgeries with greater accuracy and faster recovery times. The availability of MP1000 Robotic Surgery System varies and is subject to regulatory approvals. Please consult your local Dornier representatives for the most current information.
Executive Perspective
“This expansion underscores Dornier’s commitment to lead beyond lithotripsy and laser technologies, bringing next-generation robotic solutions to urologists worldwide. It is also consistent to our mission to be a global integrated urology company,” said Wong Yau Chung, Group CEO, Advanced MedTech Group and Dornier MedTech.
“I am confident and optimistic that we will mutually benefit from this partnership and look forward to continued growth together.”
Disclaimer
This announcement may contain general information about products and services. Availability, approved indications, timelines, description of product features, and labelling of such products or services may vary and are subject to local regulatory approval in each respective market, and clinical performance and patient outcomes may differ across care settings and patient profiles. This announcement may also contain market position and forward-looking statements based on the current views and assumptions of the issuing entity. These statements are subject to known and unknown risks, uncertainties, and other factors — many of which are beyond our control, including, but not limited to, regulatory approval delays, changes in market conditions, competitive developments, technological advancements, and economic factors, that may cause actual results to differ materially from those expressed or implied, if any. These statements are provided on an “as-is” basis and are not guarantees of future performance. Accordingly, readers are cautioned not to place undue reliance on them. No obligation is assumed to update any information in this announcement, except where mandated by applicable law. This announcement is intended for informational purposes only and is not directed at, or intended for distribution to, persons in jurisdictions where such distribution would be unlawful.
About Dornier MedTech
Dornier MedTech is a global MedTech innovator in minimally invasive urology, with a proud legacy rooted in aerospace engineering and precision German manufacturing.
Part of Advanced MedTech Holdings (AMTH) and is headquartered in Germany, Dornier is recognized for pioneering extracorporeal shock wave lithotripsy (ESWL) and continues to advance the urological field as a global innovator in laser technology.
Driven by clinical integrity and an innovation pipeline, Dornier supports healthcare professionals across the globe in delivering intelligent, precision-driven care designed to enhance operational efficiency and patient care. Its flagship technologies include the Thulio® laser, and UroGPT™, one of the first AI tools dedicated to kidney stone management.
For media inquiries, you can contact:
Rachel Lee
Head of Communications, Global
Advanced MedTech Holdings
Rachel.lee@advanced-medtech.com